FDA Warns Firms for Selling Illegal E-Cigarettes That Look Like Toys, Food & Cartoon Characters
FDA News Release Companies Must Stop Marketing Unauthorized Products Or Risk Enforcement, According to FDA The U.S. Food and Drug Administration issued warning letters Nov. 16, 2022, to five firms for the unauthorized marketing of 15 different e-cigarette products. Each e-cigarette product is packaged to look like toys, food, or cartoon characters and is likely to promote use by youth. None of the manufacturers submitted a premarket...
FDA: Foundational Rule To Improve Traceability Of Contaminated Food Finalized
News Release — Nov. 15, 2022 Today, the U.S. Food and Drug Administration announced an unprecedented advancement in foodborne illness prevention through the finalization of a rule to more effectively trace contaminated food through the food supply, whether sourced in the U.S. or abroad. The final rule establishes additional traceability recordkeeping requirements for those that manufacture, process, pack or hold...
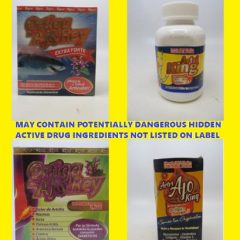
FDA Warns: Don’t Purchase or Use Artri Or Ortiga Products Due To Possible Hidden Ingredients
USFA Public Notification, Nov. 1, 2022 FDA continues to warn consumers not to purchase or use Artri and Ortiga products as the agency received adverse event reports, including of liver toxicity and death, associated with the use of Artri King and similarly named products since the first consumer warning about an Artri Ajo King product was issued on January 5, 2022, and another in April, 20, 2022. These products are...
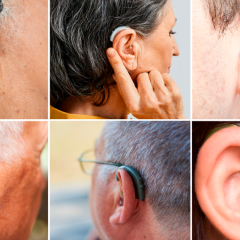
FDA Finalizes Rule Improving Access To OTC Hearing Aids
More Affordable Hearing Aids For Those With Mild To Moderate Hearing Impairment Could Be In Stores As Soon As Mid-October Today (Aug. 16, 2022), the U.S. Food and Drug Administration issued a final rule to improve access to hearing aids which may in turn lower costs for millions of Americans. This action establishes a new category of over-the-counter (OTC) hearing aids, enabling consumers with perceived mild to moderate hearing...
FDA Approves First Targeted Therapy For HER2-Low Breast Cancer
USFDA News Release: Aug. 5, 2022 Today, the U.S. Food and Drug Administration approved Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV infusion for the treatment of patients with unresectable (unable to be removed) or metastatic (spread to other parts of the body) HER2-low breast cancer. This is the first approved therapy targeted to patients with the HER2-low breast cancer subtype, which is a newly defined subset of HER2-negative...
FDA Safety Communication: Do Not Use Baby Neck Floats Due to the Risk of Death or Injury
FDA Safety Communication News Release The U.S. Food and Drug Administration (FDA) is warning parents, caregivers, and health care providers not to use neck floats with babies for water therapy interventions, especially with babies who have developmental delays or special needs, such as spina bifida, spinal muscular atrophy (SMA) type 1, Down syndrome, or cerebral palsy. The use of these products can lead to death or serious injury....
FDA Denies Authorization To Market JUUL Products
MDO: Currently Marketed JUUL Products Must Be Removed From US Market Today, the U.S. Food and Drug Administration issued marketing denial orders (MDOs) to JUUL Labs Inc. for all of their products currently marketed in the United States. As a result, the company must stop selling and distributing these products. In addition, those currently on the U.S. market must be removed, or risk enforcement action. The products include...
FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
FDA Authorizes First COVID-19 Test Available Without Prescription That Also Detects Flu, RSV
The EUA Authorizes At-Home Sample Collection With Testing Performed In A Laboratory FDA NEWS RELEASE – Monday, May 16, 2022 Today, the U.S. Food and Drug Administration authorized the Labcorp Seasonal Respiratory Virus RT-PCR DTC Test for use without a prescription by individuals with symptoms of respiratory viral infection consistent with COVID-19. This product is the first direct-to-consumer (non-prescription) multi-analyte...
FDA Approves Novel, Dual-Targeted Treatment For Type 2 Diabetes
USFDA News Release – May 13, 2022 In Clinical Trials, Treatment Proved More Effective Than Other Therapies Evaluated May 13, 2022, the U.S. Food and Drug Administration approved Mounjaro (tirzepatide) injection to improve blood sugar control in adults with type 2 diabetes, as an addition to diet and exercise. Mounjaro was effective at improving blood sugar and was more effective than the other diabetes therapies with which it...