
FDA continues to warn consumers not to purchase or use Artri and Ortiga products as the agency received adverse event reports, including of liver toxicity and death, associated with the use of Artri King and similarly named products since the first consumer warning about an Artri Ajo King product was issued on January 5, 2022, and another in April, 20, 2022. These products are promoted with unproven claims to treat arthritis and osteoarthritis, restore cartilage, and stop joint deterioration, according to a FDA public notice.
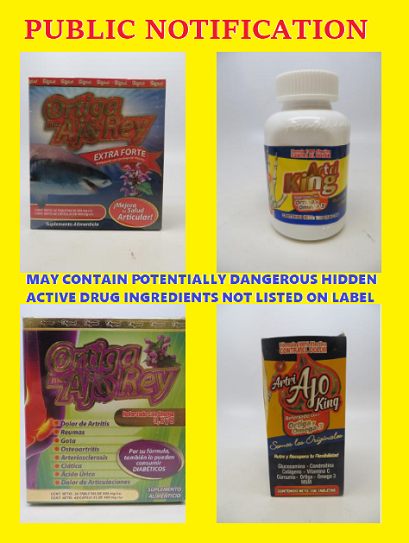
FDA analysis revealed certain Artri and Ortiga products contain hidden drug ingredients, including corticosteroids. Undeclared drug ingredients found include:
- Dexamethasone (a corticosteroid) that can cause serious adverse events, including infections, increased blood glucose (sugar) levels, changes in blood pressure, damage to bones, psychiatric problems, and adrenal dysfunction;
- Diclofenac sodium (an anti-inflammatory drug) that can lead to adverse cardiovascular events, such as heart attack and stroke, or serious gastrointestinal damage, including bleeding, ulceration, and fatal tears of the stomach and intestines, or liver toxicity including liver failure that can cause the need for a liver transplant or death
- Methocarbamol (a muscle relaxant) that can cause sedation, dizziness, and low blood pressure.
These drug ingredients, which are not listed on the product label, can also interact with other drugs a consumer is taking.
Suddenly stopping corticosteroids after long-term use or high doses can result in a serious withdrawal syndrome that includes fatigue, nausea, low blood pressure, low blood glucose levels, fever, dizziness, muscle and joint pain, and shortness of breath. Medical intervention may be necessary.
In support of public safety, and because some of these products may still be available on the market, FDA issued warning letters on October 28, 2022, to Amazon, Walmart, and Latin Foods Market for distributing various “Artri” and/or “Ortiga” unapproved and misbranded drug products. Walmart and Latin Foods Market already issued voluntary recalls for these products, the FDA notice states.
Customers with questions about these products should discuss appropriate treatment options with their health care providers.
FDA encourages health professionals and patients to report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program:
Click here to download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on form, or submit by fax to 1-800-FDA-0178.
The FDA analyses reflect only the undeclared ingredients discovered in one product from a specific lot, but ingredients may vary from product to product or from lot to lot. Products marketed as dietary supplements that are found to have hidden drug ingredients generally fail to comply with most current good manufacturing practices designed to ensure product quality and safety. Therefore, consumers should expect the manufacturing processes for Artri and Ortiga products are unreliable in providing consistent amounts of active ingredients or to prevent the introduction of unknown chemicals or other impurities.
The FDA is investigating the distribution of these products in the United States and has advised certain companies not to sell or distribute these products. The agency may take additional enforcement steps that may include warning letters, seizure, injunction, or criminal charges.





