Steps FDA Is Taking to Improve Specialty, Infant Formula Supply
FDA News Release Today (May 10, 2022), the U.S. Food and Drug Administration is providing an update on its work to increase the availability of infant and specialty formula products. On Feb. 17, the agency warned consumers not to use certain powdered infant formula products from Abbott Nutrition’s Sturgis, Michigan facility, and Abbott initiated a voluntary recall of certain products. Since that time, the agency has been working with...
FDA Permits Marketing For New Test To Improve Diagnosis Of Alzheimer’s Disease
The U.S. Food and Drug Administration today (Wednesday, May 4, 2022) permitted marketing for the first in vitro diagnostic test for early detection of amyloid plaques associated with Alzheimer’s disease. The Lumipulse G β-Amyloid Ratio (1-42/1-40) test is intended to be used in adult patients, aged 55 years and older, presenting with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive...
FDA Proposes Product Standards Prohibiting Menthol Cigarettes, Flavored Cigars
Administration Hopes Change Will Help Prevent Youth Use, Significantly Reduce Tobacco-Related Disease and Death US. Food And Drug Administration April 28, 2022 Release Today (April 28, 2022), the U.S. Food and Drug Administration is announcing proposed product standards to prohibit menthol as a characterizing flavor in cigarettes and prohibit all characterizing flavors (other than tobacco) in cigars. These actions have the potential...
Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment For Young Children
Today, the U.S. Food and Drug Administration expanded the approval to Gilead Sciences Inc. of the COVID-19 treatment Veklury (remdesivir) to include pediatric patients 28 days of age and older weighing at least 3 kilograms (about 7 pounds) with positive results of direct SARS-CoV-2 viral testing, who are: Hospitalized, orNot hospitalized and have mild-to-moderate COVID-19 and are at high risk for progression to severe COVID-19,...
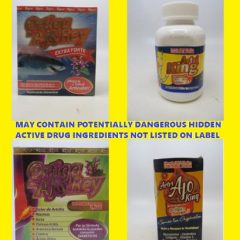
FDA Warns Consumers Artri and Ortiga Products May Contain Hidden Active Drug Ingredients
The FDA issued a warning Wednesday to consumers not to purchase or use products marketed with variations of the names “Artri” or “Ortiga” due to potentially dangerous hidden active drug ingredients not listed on the product label. FDA urges consumers taking these products to immediately talk to their health care professional (e.g., doctor) to safely discontinue use of the product because suddenly stopping these drugs may be dangerous....
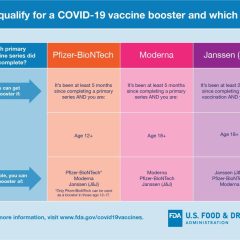
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
FDA Approves Second COVID-19 Vaccine
News Release – Monday, Jan. 31, 2022 Moderna To Be Marketed as Spikevax COVID-19 Vaccine For Ages 18+ Today (Jan. 31, 2022), the U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. Key points Spikevax meets the FDA’s rigorous...
FDA Shortens Interval For Booster Dose of Moderna COVID-19 Vaccine to 5 Months
FDA News Release – Jan. 7, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least 5 months for individuals 18 years of age and older. “The country is in the middle of a wave of the highly contagious omicron variant, which spreads more rapidly than...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...
FDA Expands Eligibility For COVID-19 Vaccine Booster For All Individuals 18 Or Older
FDA News Release – November 19, 2021 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The Centers for Disease Control and Prevention’s (CDC)...