Changes in Unemployment Rate in Texas – Updated WalletHub Study
With inflation remaining high and the U.S. gaining 223,000 jobs in December, fewer than in the previous month, WalletHub released updated rankings for its report on Changes in Unemployment Rate by State, along with a WalletHub Q&A. In order to take stock of how unemployment rates are changing throughout the U.S., WalletHub compared the 50 states and the District of Columbia based on six key metrics that compare unemployment rate...
USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...
Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
Notable Updates To Sulphur Springs ISD Handbooks, Policies
District Committee Auditing And Updating District Safety & Security Plan By now, most parents have already sign form acknowledging they’ve received and will require their students to abide by the policies and procedures listed in their student handbooks, Safe Return to In-Person Instruction and Continuity of Services Plan. Those who have more than one student may not have taken the time to flip through to see what, if any,...
COVID-19 Vaccinations For Children Ages 5-11 Offered In MMU Tent Each Morning This Week
CHRISTUS Mother Frances Hospital – Sulphur Springs Business News –January 31, 2022 By Holly Ragan, Senior Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] COVID Testing at CHRISTUS Trinity Clinics | Urgent Care Due to a shortage of COVID testing supplies, we unfortunately cannot guarantee that rapid tests will be available. If rapid tests are not available, we will be able...
FDA Approves Second COVID-19 Vaccine
News Release – Monday, Jan. 31, 2022 Moderna To Be Marketed as Spikevax COVID-19 Vaccine For Ages 18+ Today (Jan. 31, 2022), the U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. Key points Spikevax meets the FDA’s rigorous...
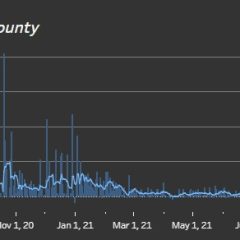
COVID-19 Continues To Rise, With 578 Active Cases In Hopkins County On Jan. 14
As has been the case across the country, state and world, COVID-19 cases have continued to surge in Hopkins County this week. Over the past four days alone, new cases have far outpaced recoveries with only 24 recoveries and 157 new COVID cases, which coupled with the 174 new cases reported Jan. 1-7, 2022, leaves 578 Hopkins County resident who still actively had COVID-19 on Thursday, Jan. 13, according to Texas Department of State...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...
FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine For Emergency Use In Children 5 Through 11 Years of Age
Friday, Oct. 29, 2021 USFDA New Release Today, the U.S. Food and Drug Administration authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include children 5 through 11 years of age. The authorization was based on the FDA’s thorough and transparent evaluation of the data that included input from independent advisory committee experts who overwhelmingly voted in favor of making the...
FDA Takes Additional Actions On Use Of A Booster Dose For COVID-19 Vaccines
US FDA News Release The U.S. Food and Drug Administration Wednesday, Oct. 20, took action to expand the use of a booster dose for COVID-19 vaccines in eligible populations. The agency is amending the emergency use authorizations (EUA) for COVID-19 vaccines to allow for the use of a single booster dose as follows: The use of a single booster dose of the Moderna COVID-19 Vaccine that may be administered at least 6 months after...