FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment For Young Children
Today, the U.S. Food and Drug Administration expanded the approval to Gilead Sciences Inc. of the COVID-19 treatment Veklury (remdesivir) to include pediatric patients 28 days of age and older weighing at least 3 kilograms (about 7 pounds) with positive results of direct SARS-CoV-2 viral testing, who are: Hospitalized, orNot hospitalized and have mild-to-moderate COVID-19 and are at high risk for progression to severe COVID-19,...
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
FDA Approves Second COVID-19 Vaccine
News Release – Monday, Jan. 31, 2022 Moderna To Be Marketed as Spikevax COVID-19 Vaccine For Ages 18+ Today (Jan. 31, 2022), the U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. Key points Spikevax meets the FDA’s rigorous...
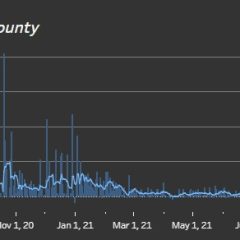
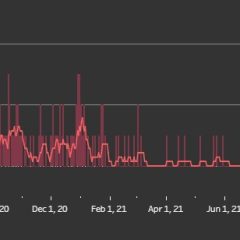
COVID-19 Continues To Rise, With 578 Active Cases In Hopkins County On Jan. 14
As has been the case across the country, state and world, COVID-19 cases have continued to surge in Hopkins County this week. Over the past four days alone, new cases have far outpaced recoveries with only 24 recoveries and 157 new COVID cases, which coupled with the 174 new cases reported Jan. 1-7, 2022, leaves 578 Hopkins County resident who still actively had COVID-19 on Thursday, Jan. 13, according to Texas Department of State...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...
Over 800 Active COVID Cases Reported For Hopkins County Over The Last 10 Days
3 Additional Hopkins County COVID Deaths Confirmed COVID cases in Hopkins County have skyrocketed over the last 10 days, going from a total of 77 new cases from Dec. 1-14 to 899 new cases reported as of Dec. 28, 2021 – 835 of those cases were reported to still be active on Tuesday. Three additional Hopkins County residents’ deaths have also been confirmed by death certificate to have been caused by COVID-19, according to...
Active COVID Case Count Doubled Over The Last 2 1/2 Weeks In Hopkins County
3 COVID Deaths Confirmed For Hopkins County In Last 45 Days Although the number of active COVID cases Texas Department of State Health Services has reported this week are lower than those reported from August through November, the active case count for Hopkins County has more than doubled in the last 2 1/2 weeks. Three additional Hopkins County residents are also confirmed to have died from COVID over the last 45 days as well,...
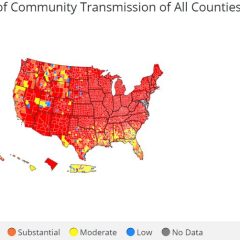
First Known Case Of COVID-19 Omicron Variant In Texas Identified In Harris County
DSHS News Release – 6:59 p.m. Monday, Dec. 6, 2021 The first known Texas case of the COVID-19 B.1.1.529 variant has been identified in a resident of Harris County. The adult female resident was recently diagnosed with COVID-19. Results of genetic sequencing this week showed that the infection was caused by the Omicron variant strain. The case is being investigated by Harris County Public Health and the Texas Department of State...
FDA Expands Eligibility For COVID-19 Vaccine Booster For All Individuals 18 Or Older
FDA News Release – November 19, 2021 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The Centers for Disease Control and Prevention’s (CDC)...