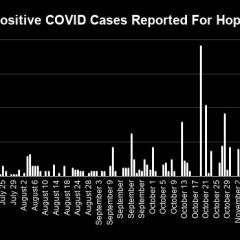
Dec. 10 COVID 19 Update: 1 Fatality, 5 New Cases
Another COVID-19 fatality was reported for Hopkins County on Thursday, along with five new COVID-19 cases and an increase in the patient count in the COVID-19 unit at CHRISTUS Mother Frances Hospital-Sulphur Springs, according to the Dec. 10 Texas Department of State Health Services COVID-19 dashboard and Hopkins County/Sulphur Springs Emergency Management officials’ Dec. 10 COVID-19 update. Dec. 10 COVID-19 Case Counts...
Meal A Day Earns Award for Excellence During COVID 19
Getting food, resources and basic services to shut-ins has been a challenge for everyone during the past six months since COVID-19 struck. On October 22, 2020, the North Texas Food Bank recognized the Meal A Day of Sulphur Springs with an Award of Excellence, in appreciation for the excellence in service demonstrated through it’s COVID-19 response. Meal a Day was recognized on October 22, 2020 Karon Weatherman, Director of the...
Mental health advocates ask Texas lawmakers to replace expiring COVID-19 relief funding
By Stephen Simpson, The Texas Tribune – Jan. 2, 2025 “Mental health advocates ask Texas lawmakers to replace expiring COVID-19 relief funding” was first published by The Texas Tribune, a nonprofit, nonpartisan media organization that informs Texans — and engages with them — about public policy, politics, government and statewide issues. Replacing federal pandemic relief funding critical to community programs could...
FDA Approves First Oral Treatment For COVID-19 In Adults
May 26, 2023 – Today, the U.S. Food and Drug Administration approved the oral antiviral Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is the fourth drug—and first oral antiviral pill—approved by the FDA to treat COVID-19 in adults....
USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...
Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
Updated COVID-19 Booster Vaccines Expected To Be Available In Texas This Week
DSHS News Release With updated COVID-19 booster vaccines now authorized and recommended for use, doses are expected to begin shipping over the next few days and arriving at health care providers across Texas this week. The Centers for Disease Control and Prevention has allocated a total of about 900,000 doses of the updated boosters to Texas, including 502,500 doses of the Pfizer vaccine and 192,800 doses of the Moderna vaccine...
COVID-19 Education and Awareness Panel to be Held May 21st
A COVID-19 Education and Awareness Panel, presented by The University of Texas at Tyler, Ben and Maytee Fisch with the College of Pharmacy, and Care Wellness, will be held May 21st, 10am to 1pm, at MLK Drive Church of Christ. Come join the discussion with healthcare professions about the fears and doubts of COVID-19 vaccines. Vaccines will be provided. COVID 19 Education and Awareness Panel
FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
FDA Authorizes First COVID-19 Test Available Without Prescription That Also Detects Flu, RSV
The EUA Authorizes At-Home Sample Collection With Testing Performed In A Laboratory FDA NEWS RELEASE – Monday, May 16, 2022 Today, the U.S. Food and Drug Administration authorized the Labcorp Seasonal Respiratory Virus RT-PCR DTC Test for use without a prescription by individuals with symptoms of respiratory viral infection consistent with COVID-19. This product is the first direct-to-consumer (non-prescription) multi-analyte...