COVID-19 Vaccine Clinic Time Change, Shortage Of Testing Supplies Reported For CHRISTUS Facilities
Holly Ragan, Senior Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] COVID Testing at CHRISTUS Trinity Clinics | Urgent Care COVID Test Due to a shortage of COVID testing supplies, we unfortunately cannot guarantee that rapid tests will be available. If rapid tests are not available, we will be able to send out a COVID PCR test, which typically results in 2 days, but could take up...
CMFH-SS Hosting Another Pfizer COVID-19 Vaccine Clinic
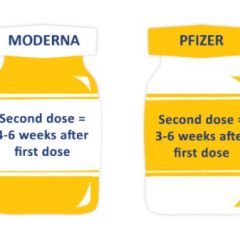
CHRISTUS Mother Frances Hospital-Sulphur Springs is hosting another Pfizer COVID-19 vaccine clinic for those individuals who have yet to have any doses of COVID-19 vaccine. The first Pfizer shot will be administered April 28 and the second dose May 19 in the lobby at the hospital. The Pfizer COVID-19 vaccine is now open to individuals age 16 and older. Registration is required to receive the first dose of Pfizer vaccine on April 28....
Appointments For The Second Dose Announced For Those Attending Recent COVID-19 Vaccine Clinics
NEWS RELEASE Appointments for the second dose of the COVID-19 vaccine for those individuals who received the first dose of the COVID-19 vaccine at one of the three clinics hosted last month in Hopkins County or the clinic held Delta County have been announced by Hopkins County/Sulphur Springs Emergency Management officials Saturday. The first follow up is slated next week. “If you had your first dose at any of the vaccination...
COVID-19 Vaccine Clinic Offered March 29; Registration Required
For those who still want to receive the COVID-19 vaccine, but were unable to register for the two-dose Moderna COVID-19 vaccine Thursday at the hospital or during shot administrations, the state will be hosting another COVID-19 vaccine clinic from 9 a.m. to 1 p.m. this coming Monday, March 29, in Sulphur Springs at the Hopkins County Civic Center. The vaccine will be the Pfizer, also a two-dose vaccine which requires a second dose in...
COVID-19 Vaccine Clinic To Be Held Saturday In Sulphur Springs; Registration Required
This Saturday, March 20, 2021, from 9 a.m. to 1 p.m. the state will be hosting a COVID-19 vaccine clinic in Sulphur Springs at the First United Methodist Church Gymnasium, Sulphur Springs Police Department Chief and Emergency Management Coordinator Jason Ricketson announced Tuesday afternoon. “At this time, we are still vaccinating under category 1B to include teachers and first responders. To register for the vaccine you can...
COVID-19 Vaccines Now Available At Cody Drug, April 28 Clinic At Hospital
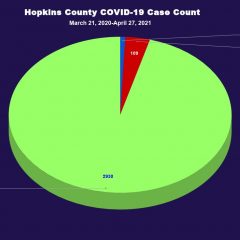
109th COVID-19 Hopkins County Fatality Reported While the number of new COVID-19 cases reported in Hopkins County continues to dwindle as more COVID-19 vaccines become available, the virus has not been eradicated in Hopkins County. Not only are people still contracting the virus, one additional Hopkins County resident’s death has been confirmed to be the result of COVID-19 — the 109th COVID-19 fatality reported for Hopkins...
CHRISTUS Trinity Clinic Will Be Scheduling COVID-19 Vaccine Appointments Starting Jan. 19
ALL COVID-19 Vaccine Registration Will Be Conducted On The CHRISTUS Website CHRISTUS Trinity Clinic around 1 p.m. Jan. 19 received 700 doses of COVID-19 vaccine allocated to Sulphur Springs, CHRISTUS Mother Frances Hospital-Sulphur Springs CEO Paul Harvey said during a 2 p.m. Zoom press conference. Holly Ragan, Paul Harvey People meeting 1A and 1B criteria –that is healthcare workers and first responders, people age 65 and...
Low Vaccine Rates Blamed for West Texas Measles Surge
February 11, 2025 – In late January 2025, health officials in Gaines County, West Texas, reported an outbreak of measles, with 15 confirmed cases predominantly among school-aged children. Gaines County is noted for its high rate of vaccine exemptions; nearly 1 in 5 incoming kindergartners in the 2023-24 school year had not received the measles-mumps-rubella (MMR) vaccine. The initial cases were identified in unvaccinated...
FDA Approves First Oral Treatment For COVID-19 In Adults
May 26, 2023 – Today, the U.S. Food and Drug Administration approved the oral antiviral Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is the fourth drug—and first oral antiviral pill—approved by the FDA to treat COVID-19 in adults....
USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...