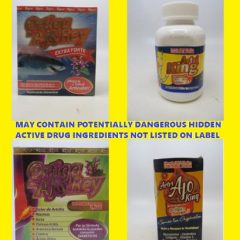
FDA Warns Consumers Artri and Ortiga Products May Contain Hidden Active Drug Ingredients
The FDA issued a warning Wednesday to consumers not to purchase or use products marketed with variations of the names “Artri” or “Ortiga” due to potentially dangerous hidden active drug ingredients not listed on the product label. FDA urges consumers taking these products to immediately talk to their health care professional (e.g., doctor) to safely discontinue use of the product because suddenly stopping these drugs may be dangerous....
FDA Warns: Don’t Purchase or Use Artri Or Ortiga Products Due To Possible Hidden Ingredients
USFA Public Notification, Nov. 1, 2022 FDA continues to warn consumers not to purchase or use Artri and Ortiga products as the agency received adverse event reports, including of liver toxicity and death, associated with the use of Artri King and similarly named products since the first consumer warning about an Artri Ajo King product was issued on January 5, 2022, and another in April, 20, 2022. These products are...