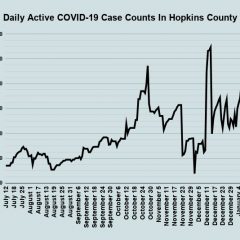
Jan. 22 COVID-19 Update: 17 New Cases, 56 Additional Vaccines Administered, COVID Hospitalizations Decrease
Although nearly twice as many new COVID-19 cases were reported on Friday than on Thursday, the COVID-19 hospitalizations decreased both locally and across the region, according to the Jan. 22 COVID-19 reports from Texas Department of State Health Services and Hopkins County/Sulphur Springs Emergency Management. While there were no new recoveries reported on Friday, there also were no new fatalities announced, but 56 additional doses...
Hopkins County COVID-19 Hospitalizations Pass 30% As Year Comes To End
In a grim reminder of how awful 2020 has been, the latest report from the Hopkins County Emergency Management Team showed 30 Covid-19 patients are currently in the Covid Unit at CHRISTUS Mother Frances Hospital in Sulphur Springs. With 96 staffed hospital beds at the local campus, that pushes the Covid Hospitalization Rate locally up to 31.25%. The Texas Department of State Health Services tracks this hospitalization rate but as a...
FDA Approves First Oral Treatment For COVID-19 In Adults
May 26, 2023 – Today, the U.S. Food and Drug Administration approved the oral antiviral Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is the fourth drug—and first oral antiviral pill—approved by the FDA to treat COVID-19 in adults....
USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...
Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment For Young Children
Today, the U.S. Food and Drug Administration expanded the approval to Gilead Sciences Inc. of the COVID-19 treatment Veklury (remdesivir) to include pediatric patients 28 days of age and older weighing at least 3 kilograms (about 7 pounds) with positive results of direct SARS-CoV-2 viral testing, who are: Hospitalized, orNot hospitalized and have mild-to-moderate COVID-19 and are at high risk for progression to severe COVID-19,...
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
13 Hopkins County COVID Deaths So Far In 2022, 166 Since March 2020
While many states and countries are lowering and, in some cases, even eliminating COVID-19 restrictions altogether, the virus has surged in other areas. After almost 2 full years, many have become weary of the extra precautions, whether required vaccinations, frequent testing, booster shots, masking, lockdowns, restricted gatherings or frequent sanitizing and extra cleansing with virus-killing substances. Health care and educational...
FDA Approves Second COVID-19 Vaccine
News Release – Monday, Jan. 31, 2022 Moderna To Be Marketed as Spikevax COVID-19 Vaccine For Ages 18+ Today (Jan. 31, 2022), the U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. Key points Spikevax meets the FDA’s rigorous...