USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...
Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
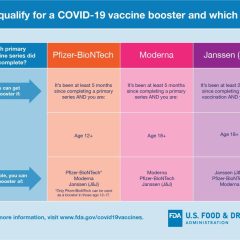
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
FDA Approves Second COVID-19 Vaccine
News Release – Monday, Jan. 31, 2022 Moderna To Be Marketed as Spikevax COVID-19 Vaccine For Ages 18+ Today (Jan. 31, 2022), the U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. Key points Spikevax meets the FDA’s rigorous...
COVID-19 Vaccine Clinic Time Change, Shortage Of Testing Supplies Reported For CHRISTUS Facilities
Holly Ragan, Senior Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] COVID Testing at CHRISTUS Trinity Clinics | Urgent Care COVID Test Due to a shortage of COVID testing supplies, we unfortunately cannot guarantee that rapid tests will be available. If rapid tests are not available, we will be able to send out a COVID PCR test, which typically results in 2 days, but could take up...
CHRISTUS Mother Frances Hospital – Sulphur Springs And NETX Public Health District Partner To Offer Pediatric COVID-19 Vaccines
Holly Ragan, Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] Sulphur Springs, Texas, January 25, 2022 – CHRISTUS Mother Frances Hospital – Sulphur Springs will be partnering with Northeast Texas Public Health District to provide COVID vaccinations for children ages 5 through 11. The vaccine clinic will be held in the MMU tent located in the parking lot north of the hospital...
FDA Shortens Interval For Booster Dose of Moderna COVID-19 Vaccine to 5 Months
FDA News Release – Jan. 7, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least 5 months for individuals 18 years of age and older. “The country is in the middle of a wave of the highly contagious omicron variant, which spreads more rapidly than...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...
FDA Expands Eligibility For COVID-19 Vaccine Booster For All Individuals 18 Or Older
FDA News Release – November 19, 2021 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The Centers for Disease Control and Prevention’s (CDC)...
FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine For Emergency Use In Children 5 Through 11 Years of Age
Friday, Oct. 29, 2021 USFDA New Release Today, the U.S. Food and Drug Administration authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include children 5 through 11 years of age. The authorization was based on the FDA’s thorough and transparent evaluation of the data that included input from independent advisory committee experts who overwhelmingly voted in favor of making the...