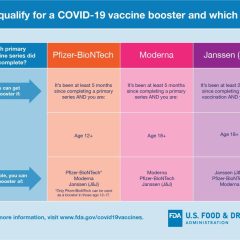
COVID Booster Shots Are Now Available
CHRISTUS Mother Frances Hospital – Sulphur Springs Business News – September 21, 2021 CHRISTUS Mother Frances Hospital – Sulphur Springs has ONE mission: To Extend the Health Ministry of Jesus Christ. By Holly Ragan, Senior Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] Do You Need a COVID test, but are unable to get in to see your provider for testing or an...
Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
Updated COVID-19 Booster Vaccines Expected To Be Available In Texas This Week
DSHS News Release With updated COVID-19 booster vaccines now authorized and recommended for use, doses are expected to begin shipping over the next few days and arriving at health care providers across Texas this week. The Centers for Disease Control and Prevention has allocated a total of about 900,000 doses of the updated boosters to Texas, including 502,500 doses of the Pfizer vaccine and 192,800 doses of the Moderna vaccine...
FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
FDA Shortens Interval For Booster Dose of Moderna COVID-19 Vaccine to 5 Months
FDA News Release – Jan. 7, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least 5 months for individuals 18 years of age and older. “The country is in the middle of a wave of the highly contagious omicron variant, which spreads more rapidly than...
FDA Expands Eligibility For COVID-19 Vaccine Booster For All Individuals 18 Or Older
FDA News Release – November 19, 2021 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The Centers for Disease Control and Prevention’s (CDC)...
The Johnson & Johnson COVID Vaccine And Booster Now Available
Sulphur Springs, Texas, October 27, 2021-CHRISTUS Mother Frances Hospital – Sulphur Springs has ONE mission: To Extend the Health Ministry of Jesus Christ. By Holly Ragan, Senior Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] No Excuses – October is Breast Cancer Awareness Month Nothing will keep us from making your breast health a priority, whether you need a mammogram,...
FDA Takes Additional Actions On Use Of A Booster Dose For COVID-19 Vaccines
US FDA News Release The U.S. Food and Drug Administration Wednesday, Oct. 20, took action to expand the use of a booster dose for COVID-19 vaccines in eligible populations. The agency is amending the emergency use authorizations (EUA) for COVID-19 vaccines to allow for the use of a single booster dose as follows: The use of a single booster dose of the Moderna COVID-19 Vaccine that may be administered at least 6 months after...
USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...