Lemonade, Tang, Tea, Kool-Aid, Hand Sanitizer, Injectables Also Recalled
Before you pour that glass of lemonade, Kool-Aid or tea, be sure to check the label. Several products have recently been recalled either voluntarily or under the direction of the US Food and Drug Administration due to the presence of potentially hazardous particles, unlabeled allergens, or possible contaminates. At least one type of candy, hummus and barbecue rub are among the products recalled due to unlabeled allergens. Certain drinks such as lemonade, tea and Kool-Aid are being recalled because of foreign particles, hand sanitizer due to type of container and a pharmaceutical injectable due to lack of sterility.
Drinks
Select code dates of Country Time Lemonade, Tang, Arizona Tea powdered beverages and limited Kool- Aid powdered beverages with “Best When Used By” dates between May 10, 2023 and November 1, 2023 are being voluntarily recalled in the U.S. due to the potential presence of foreign material, specifically very small pieces of metal or glass, that may have been introduced during production.
Additionally, select code dates of Country Time Lemonade with “Best When Used By” date of September 15, 2023 and select Tang powdered beverages with “Best When Used By” dates of August 20-21, 2023 are being voluntarily recalled in Canada for the same issue.
The issue was first discovered during an internal review at the manufacturing facility. The Company is actively working with retail partners and distributors to remove potentially impacted product from circulation.
Consumers who purchased these items, listed below, should not consume the product and can either return it to the store where it was purchased, or discard it. Consumers can contact Kraft Heinz from 9 am-6 pm Eastern Standard Time, Monday- Friday at the below phone numbers to see if a product they purchased is part of the voluntary recall and to receive reimbursement:
U.S. Consumer Relations at 1-855-713-9237 Canada Consumer Relations at 1-855-268-1775
No other sizes, varieties or code dates of Country Time Lemonade, Kool-Aid, Arizona Tea, Tang or other powdered beverages, ready-to-drink beverages or Kraft Heinz products are included in this recall.
Kraft Heinz is committed to upholding the highest safety and quality standards.
Product Size Name of Product Individual Package Best Before Date Individual Package UPC 73 oz Arizona Arnold Palmer 08/05/23 through 09/10/23 043000086643 82.5oz Country Time Lemonade 8/6/2023 through 10/22/23 043000928608 63 oz Country Time Lemonade 8/8/2023 through 9/6/23 043000082195 58.9 oz Tang Orange 8/11/2023 through 10/04/23 043000082171 63oz. Kool- Aid Tropical Punch 8/12/2023 through 9/22/23 043000082164 82.5oz. Kool- Aid Tropical Punch 8/18/2023 through 9/22/23 043000957400 82.5oz Kool-Aid Tropical Punch 8/17/2023 through 10/05/23 043000957400 82.5oz Country Time Pink Lemonade 8/20/2023 through 9/27/23 043000928615 82.5oz Country Time Pink Lemonade 8/20/23 043000928615 82.5oz Country Time Pink Lemonade 9/17/23 043000928615 72 oz Tang Orange 8/21/2023 through 9/20/23 043000032268 72 oz Tang Orange 8/22/2023 through 9/28/23 043000032268 82.5 oz 82.5Z Country Time HLF&HLF 34 QT/6 8/24/23 043000046012 82.5 oz Kool- Aid Tropical Punch 8/31/2023 through 10/06/23 043000957400 63 oz Country Time Pink Lemonade 9/6/2023 through 9/7/23 043000082188 63 oz Kool-Aid Strawberry Cherry 10/2/23 043000082201 20 oz Kool- Aid Raspberry Lemonade 8/5/2023 through 10/18/23 043000954072 20 oz Tang Orange 8/6/2023 through 10/23/23 043000032275 20 oz Tang Orange 5/15/2023 through 10/22/23 043000032275 19 oz Kool-Aid Cherry 8/9/23 043000953532 18 oz Tang Guava Pineapple 8/13/23 043000064511 19 oz Country Time Lemonade 8/11/2023 through 09/11/23 043000951170 19 oz Country Time Pink Lemonade 8/30/2023 through 9/13/23 043000951149 19 oz Kool- Aid Tropical Punch 6/12/2023 through 10/20/23 043000953501 19 oz 19Z Countrty Time HALF&HALF 8QT/12 9/13/2023 through 9/14/23 043000046005 19oz Kool-Aid Tropical Punch 8/31/2023 through 10/19/23 043000953501 19 oz Kool-Aid Strawberry 10/18/2023 through 10/19/23 043000953556 6.7 oz Country Time “on the go” Lemonade 10 pack 9/20/2023 through 10/04/23 43000010983 6.6 oz Kool -Aid Tropical Punch “on the go” 10 pack 10/19/2023 through 11/01/23 043000023464 63oz. 63oz Kool- Aid Twin pack Tropical Punch 8/16/2023 through 8/17/23 043000089712 29 oz Country Time Lemonade 12 qt 8/10/23 043000951194 116oz. Country Time Powdered Soft Drink Sugar Sweetened
Lemonade, pack of 45/10/2023 through 8/10/23 043000075388 19 oz Country Time Lemonade Drink Mix, 6 pack case 6/20/2023 through 8/12/23 043000951170 2.4 kg Country Time Original Lemonade 9/15/23 661880533800 2.2 kg Tang Orange 8/20/2023 through 8/21/23 661880575900 82.5 oz EXPORT 82.5Z SSKA TROP PNCH 6 8/18/23 4300001464 20 oz EXPORT 20Z TANG ORNG 6QT 12 8/9/23 430000322700 19 oz EXPORT 19Z KA CHRY SS 8QT KOREA EXP 12 8/8/2023 through 8/9/23 430000341600 19 oz EXPORT 19Z CT HALF&HALF 8QT/12 9/14/23 430000460000 82.5 oz EXPORT 82.5Z CT HLF&HLF 34 QT/6 8/24/23 430000460100 19 oz EXPORT 19Z SSKA TROP PNCH 12 9/5/2023 through 9/6/23 430009535000
Hummus
TaDah! Foods of Springfield, VA, is voluntarily recalling two batches of production of Spicy Brown Sugar Harissa Hummus due to an undeclared milk allergen. People who have an allergy or severe sensitivity to milk run the risk of serious or life-threatening allergic reaction if they consume these products. There have been no reports of illness to date.
The specific products being recalled are:
- TaDah!, Spicy Brown Sugar Harissa Hummus Falafel Wrap 7.5oz with a lot code 0601TaDah!, Spicy Brown Sugar Harissa Hummus Falafel Wrap 7.5oz with a “Best By” date of September 01, 2022 (090122) UPC number 85019800307-5
- TaDah!, Spicy Brown Sugar Harissa Hummus Falafel Wrap 7.5oz with a lote code 0621TaDah!, Spicy Brown Sugar Harissa Hummus Falafel Wrap 7.5oz with a “Best By” September 03, 2022 (090322) UPC number 8-5019800307-5

The company understands that this is a problem, and as an abundance of caution it is taking these measures to ensure the safety of its consumers. Consumers who have purchased the potentially affected products with these code dates are asked to immediately dispose of them and should please contact TaDah! Foods s for a full replacement or refund.
TaDah! Foods is also working with their distribution network to immediately remove these specific code-dated falafel wraps from their warehouses and from retail shelves. The falafel wraps are sold at natural food stores and grocery retailers nation-wide.
Consumers requesting refunds or with additional questions can email TaDah! Foods at [email protected] or call (571) 335-1635, Monday – Friday 9am-5pm Eastern Standard Time.
Hand Sanitizer
American Screening LLC of Shreveport, Louisiana is voluntarily recalling 153,336 units of Hand Sanitizer, containing 70% ethyl alcohol gel to the consumer level. The hand sanitizer is packaged in 8 oz. containers that resemble water bottles posing a risk of consumption.

Ingesting hand sanitizer, which is intended for topical use, could potentially result in alcohol toxicity. Symptoms of alcohol toxicity may range from lack of coordination, slowed or slurred speech, drowsiness to coma, which can be fatal. Furthermore, ingesting alcohol can affect the brain and cause impaired driving or operating heavy machinery. Alcohol can also interact with numerous drugs which may result in serious adverse effects. Ingesting alcohol by people with alcohol addiction may interfere with maintaining abstinence. Additionally, people with alcohol addiction may seek large amounts of ethanol-based hand sanitizers as a substitute. To date, American Screening LLC has not received any reports of adverse events related to this recall.
The product is intended to be applied topically to help reduce bacteria on the skin that could cause diseases when soap and water are not available and is packaged in an eight ounce bottle. The affected hand sanitizer lots include the following expiration dates of 5/21/2022 and 05/24/2022 for black capped bottles and no lot numbers or expiration dates for clear capped bottles. The product can be identified by its shape (small water bottle), with a black flip top cap or clear cap with blue pouring spout with the weight of measure of eight (8) ounces/237mL, UPC 8 4005051579 2. The Hand Sanitizer was sold to customers & distributors Nationwide via the internet at americanscreeningcorp.com and/or in-house sales.

American Screening LLC is notifying its distributors and customers by mass email and is arranging for return of all recalled Hand Sanitizers.
Consumers & distributors that have this hand sanitizer which is being recalled should stop distribution/use and return to place of purchase or discard.
Consumers with questions regarding this recall can contact Wendy Laskowski by e-mailing [email protected] or call 318-606-6037 Monday–Friday, 8:00 am–5:00 pm CST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to ingesting the hand sanitizer packaged in the 8 oz. water bottle style.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
Candy

Cargill is voluntarily recalling 469, one-pound bags of Wilbur Dark Chocolate Triple Covered Malted Milk Balls. Sold locally through the Wilbur Chocolate Store in Lititz, Pa. and online at Wilburbuds.com to customers nationwide between Sept. 28 and Nov. 17, 2021, the bags are being recalled because it may contain undeclared peanut allergen. People who have an allergy or severe sensitivity to peanuts run the risk of serious or life-threatening allergic reaction if they consume these products.
While packaging the Wilbur Dark Chocolate Triple Covered Malted Milk Balls, a Cargill employee identified a milk-chocolate covered peanut within the malted milk balls and packaging production was immediately stopped. The bag labels do bear a “may contain peanut” statement; however, Cargill is recalling the product in an abundance of caution.
Peanut allergic individuals should not consume the Dark Chocolate Triple Covered Malted Milk Balls with lot #s 211007MB and 210917MB & UPC and Item Code 200171-16.

No other Cargill Cocoa & Chocolate or Wilbur products were reported to have been involved in this recall. No illnesses or health-related complaints have been reported to date to Cargill in connection with this recall.
Customers who purchased any of these products should not consume them and should contact the Wilbur Chocolate Store immediately to receive a full refund and confirm the product has been disposed. Customers with recalled product should contact Denise Auker by email at [email protected] or by phone at 717-627-7946 between 9 a.m. and 5 p.m. Eastern, Monday-Friday.
This action by Cargill and Wilbur Chocolate is being taken for cautionary purposes and in the greatest interest of consumer safety.
Barbecue Rub
The Spice House is voluntarily recalling Sichuan Chili BBQ Rub because it may contain undeclared sesame. People who have an allergy or sensitivity to sesame seeds should immediately discontinue use of this product.
The product is being recalled because the wrong spice blend may be in the bottle and therefore it could contain undeclared sesame. Less than 40 jars of the product are affected by this issue, but all Sichuan Chili BBQ Rub produced is being recalled.
The product was only available for purchase from November 12 through November 18, 2021. It was distributed at The Spice House retail stores and on the company’s website. It is sold in a glass jar marked with the UPC 816328028240.
No allergic reactions have been reported to date.
Affected consumers should dispose of the product and contact [email protected] to obtain a replacement. Consumers with questions also may contact the company at [email protected].
The recall is being conducted voluntarily in cooperation with the U.S. Food and Drug Administration.

Injectables
CHICAGO, IL – November 19, 2021 – Sagent Pharmaceuticals, Inc. today announced the voluntary nationwide recall of four lots of Levetiracetam Injection, USP, (an antiepileptic drug available as a clear, colorless, sterile solution (100 mg/mL) for intravenous administration) to the user level. The lack of container closure integrity, found in reserve sample vials may result in a non-sterile product.
Risk Statement: Intravenous administration of a product intended to be sterile that is not sterile could result in serious systemic infections which may be life threatening. To date, Sagent has not received reports of any product complaints or adverse events associated with this issue.
Levetiracetam Injection, USP 500 mg per 5 mL, is used in the treatment of certain types of seizures and is packaged in a 5mL single-does vial. The Levetiracetam Injection, USP, label and affected lot numbers with Expiration Dates and NDC number can be found in the table below. Product was distributed Nationwide from March to November 2021.
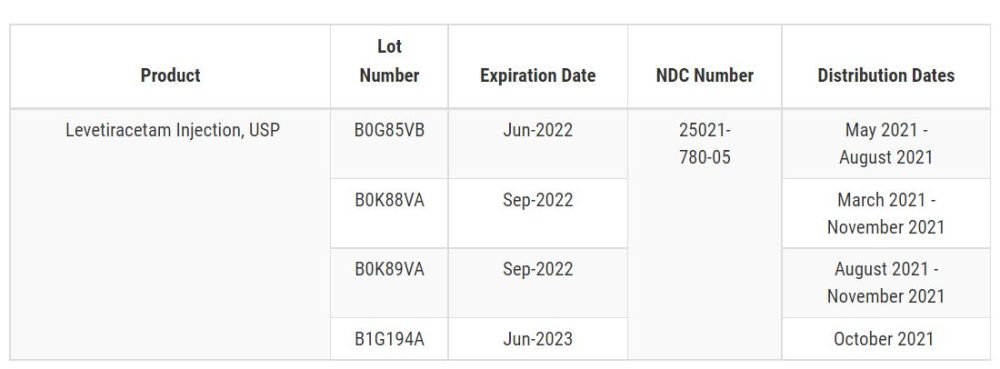
Sagent Pharmaceuticals, Inc. is notifying customers by fax, email, FedEx, and/or certified mail, which includes arrangements for return of all recalled product. Customers that have Levetiracetam Injection, USP 500mg per 5 mL, which is being recalled, have been instructed to examine their inventory immediately and to quarantine, discontinue distribution of, and return as directed the recalled lots of product. Customers who may have further distributed this product have been requested to identify their customers and notify them at once of this product recall. Healthcare/distributors/retailers that have product which is being recalled should stop using product and return the recalled product. The necessary form to document product information, as well as other information regarding this recall, is available at www.Sagentpharma.comExternal Link Disclaimer.
Consumers or healthcare workers with any questions regarding this recall can contact the customer call center (866) 625-1618 M-F, 8am-7pm CST. Patients should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.






