Time to check the fridge and cabinets to see if you have any of the products recalled over the last week due to possible cross contamination, undeclared potential allergens or high levels of some ingredients including infant rice cereal, lidocaine, dog food and trail mix.
Baby Rice Cereal
While no illnesses related to the product lots had been reported as of Oct. 8, 2021, Maple Island Inc. opted to issue a voluntary recall of three lots of its Parent’s Choice Rice Baby Cereal manufactured for Walmart. This product recall is a result of a routine sampling program by the FDA which found that a sample from three production lots of Parent’s Choice Rice Baby Cereal tested above the guidance for naturally occurring inorganic arsenic.
Maple Island Inc. conducted testing on both the raw material and finished product in question. While the test results were in compliance with the FDA’s guidelines, Maple Island Inc. is issuing this recall out of an abundance of caution. Maple Island Inc. conducted testing on both the raw material and finished product in question. While the test results were in compliance with the FDA’s guidelines, Maple Island Inc. is issuing this recall out of an abundance of caution.
This product was distributed nationally through Walmart’s stores and online. Walmart was advised and has indicated to Maple Island Inc., the product will be pulled from store shelves and a register block will be put on the product at its stores and online to prevent any further sales.
The specific 8 ounces lots of Parent’s Choice Rice Baby Cereal being recalled were sold after April 5, 2021, and include:
- Lot 21083 with UPC Code #00681131082907 with a best if used by date of JUN 24 2022.
- Lot 21084 with UPC Code #00681131082907 with a best if used by date of JUN 25 2022
- Lot 21242 with UPC Code #00681131082907 with a best if used by date of NOV 30 2022

The “Best If Used By” date and product numbers can be found in the bottom left corner on the back of the Parent’s Choice Rice Baby Cereal packaging.
Customers who may have purchased Parent’s Choice Rice Baby Cereal at Walmart with Lot Number 21083/UPC Code #00681131082907 and best if used by date of JUN 24 2022, Lot Number 21084/UPC Code #00681131082907 and best if used by date of JUN 25 2022, or Lot 21242 with UPC Code #00681131082907 with a best if used by date of NOV 30 2022 should discard the product or return it to Walmart for a full refund. Customers seeking additional information may call Maple Island Inc., Monday through Friday 8am – 4pm Central time at 1-800-369-1022 or contact the company by email at [email protected].
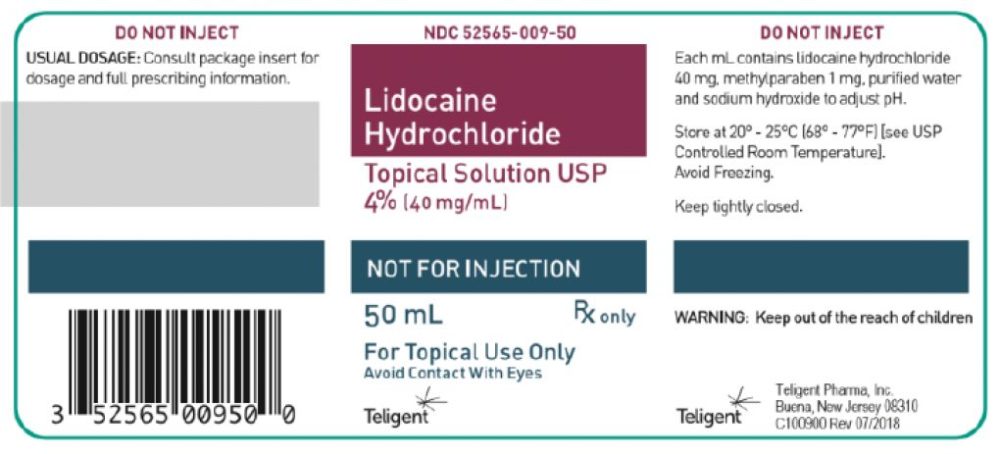
Topical Lidocaine Hydrochloride
Buena, NJ, Teligent Pharma, Inc. Tuesday, Oct. 12, announced a voluntary recall of five lots of (40 mg/mL), 50 mL crew cap glass bottles of Lidocaine HCl Topical Solution 4% at the user level because testing found the solution can be “super potent based on an Out of Specification (OOS) result obtained at the 18-month stability timepoint.” As of Tuesday, Teligent Pharma, Inc. had not received any reports of adverse events related to this recall. The product was distributed at the wholesale and retail distribution levels in the US and Canada.
Use of the super potent product would result in a higher than intended lidocaine dose above that intended. An increased lidocaine dose could lead to the development of local anesthetic systemic toxicity depending on the duration of the treatment and the specific patient. Local anesthetic systemic toxicity can result in central nervous system reactions including excitation and/or depression and more serious signs of cardiovascular toxicity, such as bradycardia, hypotension, and even cardiovascular collapse can present very quickly. If local anesthetic systemic toxicity is not recognized and treated quickly, severe morbidity and even death can result. Adults and the elderly who are more likely to use this product as well as children of lower body weight are more likely to experience local anesthetic systemic toxicity if a higher than intended lidocaine concentration is administered.NDC Lot Number Expiration 2565-009-50 13262 03/2022 14217 08/2022 13058 02/2022 13768 05/2022 63739-997-64 16306 01/2024
Teligent Pharma, Inc., is notifying its distributors via Fed-Ex and is arranging for return of all recalled products.
Consumers and patients that have Lidocaine HCl Topical Solution 4% which is being recalled are asked to discontinue use and dispose of the product immediately.
Consumers can call 1-856.697.1441 press * to reach the medical information call center Monday through Friday, 8am – 5pm or send an e-mail to [email protected] for any product or recall related questions for Lot #13262 Exp. 03/2022, Lot #14217 Exp. 08/2022, Lot #13058 Exp. 02/2022, Lot #13768 Exp. 05/2022, Lot #16306 Exp. 01/2024.
Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Refrigerated Dog Food
Tuffy’s Pet Foods on Oct. 11 issued a voluntary recall of approximately 1,600 cases of Pure Vita Salmon Entree Dog Food in a Tetrapak carton following notification from the product manufacturer of potentially elevated levels of Vitamin D. While no reports of illness or injury have been reported from consumers to date, Tuffy’s is acting out of an abundance of caution and is removing the specific lots of product listed below from distribution.
Consuming elevated levels of vitamin D in dog food can cause adverse reactions in dogs of all sizes, including symptoms such as vomiting, loss of appetite, increased thirst, increased urination, excessive drooling and weight loss. When ingested at excessive levels, vitamin D can lead to serious health issues in dogs including renal dysfunction.
Consumers should stop feeding the product listed below to their pets immediately. Tuffy’s recommends consumers whose dogs have consumed the potentially affected product and are exhibiting any of the above mentioned symptoms contact their veterinarian.Product Name Net Weight UPC Lot Numbers Best By Dates Pure Vita Salmon Entrée Dog Food TetraPak 12.5 oz. per carton 0 73893 96202 1 0629101N1
0901101N129 Jun 2023
1 Sep 2023
The recalled product was distributed exclusively within the United States to distributors and retail stores. The recalled product is limited to Pure Vita Salmon Entrée Dog Food in a Tetrapak carton, bearing UPC Code “0 73893 96202 1” (found on side of the carton). Products included in the recall are identified by the “Best by Dates” and “Lot Numbers” (found on the top of the carton) as listed in the grid below. No other Pure Vita dog or cat foods, or treats are affected by this announcement.
This product recall was initiated after Tuffy’s was notified by the product manufacturer that this product may contain elevated levels of vitamin D. The manufacturer of the affected product has identified and isolated the error and corrective actions are in progress to prevent this from happening again.
Consumers who purchased the Pure Vita Salmon Entrée dog food product subject to this voluntary recall are urged to return the product to their retailer for a full refund.
For consumer information or questions regarding this voluntary recall, please contact Tuffy’s Pet Foods, Inc. at (800) 525-9155 from Monday-Friday, 8:00am-5:00pm Central Time, or by email at [email protected].


Specialty Trail Mix
Nestlé Professional, out of Solon, Ohio on Oct 7. issued a recall of four Nature’s Heart 1.5 ounce fruit and trail mix products because they may contain undeclared peanuts. People who have an allergy to peanuts run the risk of serious or life-threatening allergic reaction if they consume these products.
The product recall was initiated after Nestlé Professional received two complaints from individuals with peanut allergies who ate the Nature’s Heart Superfoods Trail Mix and Mango Turmeric Cashew Glazed Mix and experienced mild reactions. No severe reactions or hospitalizations have been reported.
Recalled Products include:
None of the products contain peanuts, and peanuts are not identified as an allergen on the product labels, but we are investigating whether the products may have been inadvertently cross-contaminated with peanuts during manufacture.
The product recall only applies to the four Nature’s Heart products (listed above) sold in 1.5 oz packages. No other retail Nature’s Heart products are affected.
Consumers who have purchased these products are urged not to consume them. They should be thrown away or returned to the place of purchase.
Retailers and consumers with questions may call Nestlé Professional Customer Service at 800-288-8682.
- Nature’s Heart 1.5 oz Superfood Trail Mix
- Pouch UPC: 050000211944, Case UPC: 050000618569
- DEC 2021 Best By Date/ Batch Codes: 1083T353T2, 1084T353T2, 1085T353T2, 1086T353T2, 1088T353T2, 1089T353T2
- APR 2022 Best By Date/ Batch Code: 1200T353T3



- Nature’s Heart 1.5 oz Toasted Coconut Chips
- Pouch UPC: 050000695454, Case UPC: 050000695454
- JAN 2022 Best By Date/ Batch Code: 1120T353T2
- FEB 2022 Best By Date/ Batch Codes: 1121T353T2, 1123T353T2, 1124T353T2
- APR 2022 Best By Date/ Batch Code: 1197T353T3



- Nature’s Heart 1.5 oz Pineapple Chili Cashew Glazed Mix
- Pouch UPC: 050000867967, Case UPC: 050000948758
- JAN 2022 Best By Date/ Batch Codes: 1096T353T2, 1097T353T2
- FEB 2022 Best By Date/ Batch Codes: 1140T353T2, 1141T353T2, 1144T353T2, 1145T353T2



- Nature’s Heart 1.5 oz Mango Turmeric Cashew Glazed Mix
- Pouch UPC: 050000891450, Case UPC: 050000692514
- DEC 2022 Best By Date/ Batch Codes: 1089T353T2, 1090T353T2
- JAN 2022 Best By Date/Batch Code: 1091T353T2










