Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
Alcohol Isn’t The Only Thing A Breath Test Can Detect; It Can Now Detect COVID-19 Too
Alcohol isn’t the only thing a breath test can detect; COVID-19 can now be detected in less than 3 minutes using a breath sample. Not having to stick a long cotton swab up the nose, have a blood sample drawn or cheek swab to check for the virus sounds great, just don’t expect to see the new diagnostic devices readily available to the average consumer anytime soon – or even some locations that previously conducted...
FDA Approves Second COVID-19 Vaccine
News Release – Monday, Jan. 31, 2022 Moderna To Be Marketed as Spikevax COVID-19 Vaccine For Ages 18+ Today (Jan. 31, 2022), the U.S. Food and Drug Administration approved a second COVID-19 vaccine. The vaccine has been known as the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. Key points Spikevax meets the FDA’s rigorous...
Jan. 12 Deadline For Rural Hospitals, Nursing Facilities To Apply For ARPA COVID-19 Funding
AUSTIN – The Texas Health and Human Services Commission is reminding rural hospitals and nursing facilities in Texas to apply for $128 million in federal American Rescue Plan Act funding to help pay for critical staffing needs during the COVID-19 pandemic. The deadline to apply is Wednesday, Jan. 12. “We’re encouraging health care providers who have worked so hard in responding to the COVID-19 pandemic to take advantage of this...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...
First Known Case Of COVID-19 Omicron Variant In Texas Identified In Harris County
DSHS News Release – 6:59 p.m. Monday, Dec. 6, 2021 The first known Texas case of the COVID-19 B.1.1.529 variant has been identified in a resident of Harris County. The adult female resident was recently diagnosed with COVID-19. Results of genetic sequencing this week showed that the infection was caused by the Omicron variant strain. The case is being investigated by Harris County Public Health and the Texas Department of State...
FDA Expands Eligibility For COVID-19 Vaccine Booster For All Individuals 18 Or Older
FDA News Release – November 19, 2021 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUA) for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. The Centers for Disease Control and Prevention’s (CDC)...
FDA Takes Additional Actions On Use Of A Booster Dose For COVID-19 Vaccines
US FDA News Release The U.S. Food and Drug Administration Wednesday, Oct. 20, took action to expand the use of a booster dose for COVID-19 vaccines in eligible populations. The agency is amending the emergency use authorizations (EUA) for COVID-19 vaccines to allow for the use of a single booster dose as follows: The use of a single booster dose of the Moderna COVID-19 Vaccine that may be administered at least 6 months after...
Divorce Rates Drop as Pandemic Continues
By Johanna Hicks, Texas A&M AgriLife Extension, Family & Community Health Agent, Hopkins County As Family & Community Health Extension Agent for Texas A&M AgriLife Extension, an article recently caught my eye. I am a strong advocate for traditional marriage relationships. Strong marital relationships lead to strong families and strong families lead to strong communities. The article was part of an...
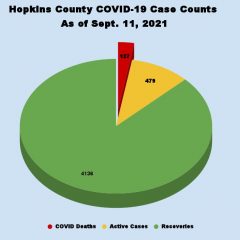
More Hopkins County COVID-19 Cases Reported During The Last 2 Weeks Than June and July Combined
Another Hopkins County Resident Confirmed To Have Died From Coronavirus Coronavirus cases continue to surge, with more new COVID-19 cases reported among Hopkins County residents during the last 2 weeks than during June and July combined. Two of the 461 Hopkins County residents who’ve had COVID during the last 2 weeks were confirmed by death certificate to have died as a result of the virus; the most recent COVID death was one of...