FDA Approves First Oral Treatment For COVID-19 In Adults
May 26, 2023 – Today, the U.S. Food and Drug Administration approved the oral antiviral Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is the fourth drug—and first oral antiviral pill—approved by the FDA to treat COVID-19 in adults....
FDA Authorizes First COVID-19 Test Available Without Prescription That Also Detects Flu, RSV
The EUA Authorizes At-Home Sample Collection With Testing Performed In A Laboratory FDA NEWS RELEASE – Monday, May 16, 2022 Today, the U.S. Food and Drug Administration authorized the Labcorp Seasonal Respiratory Virus RT-PCR DTC Test for use without a prescription by individuals with symptoms of respiratory viral infection consistent with COVID-19. This product is the first direct-to-consumer (non-prescription) multi-analyte...
Social Security Offices To Resume In-Person Services Beginning April 7, 2022
Online, Phone Still Most Recommended Ways To Contact SSA For Assistance, To Schedule Appointments, But Not Required Social Security Administration Acting Commissioner Kilolo Kijakazi just announced good news for those individuals in need of assistance from the agency: local officers will be resuming in-person services this week. Social Security Administration logo “I am pleased to announce that local Social Security offices will...
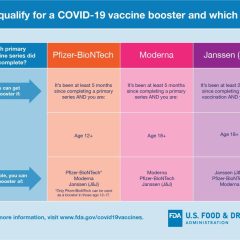
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
Jan. 12 Deadline For Rural Hospitals, Nursing Facilities To Apply For ARPA COVID-19 Funding
AUSTIN – The Texas Health and Human Services Commission is reminding rural hospitals and nursing facilities in Texas to apply for $128 million in federal American Rescue Plan Act funding to help pay for critical staffing needs during the COVID-19 pandemic. The deadline to apply is Wednesday, Jan. 12. “We’re encouraging health care providers who have worked so hard in responding to the COVID-19 pandemic to take advantage of this...
FDA Shortens Interval For Booster Dose of Moderna COVID-19 Vaccine to 5 Months
FDA News Release – Jan. 7, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least 5 months for individuals 18 years of age and older. “The country is in the middle of a wave of the highly contagious omicron variant, which spreads more rapidly than...
Pfizer COVID-19 Vaccine Approved By FDA
The U.S. Food and Drug Administration on Monday fully approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 disease in individuals 16 years of age and older. Pfizer-BioNTech COVID-19 Vaccine receives full FDA approval The vaccine will also continue to be available under emergency use...
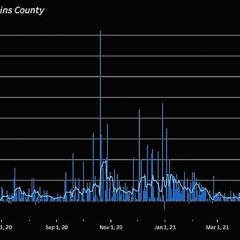
Active COVID-19 Case Count In Hopkins County On The Rise
As has been the case across the state and country, the active COVID-19 case county in Hopkins County rose significantly over the last week, but, unlike in some larger cities where hospitals are having to divert COVID-19 patients to other healthcare facilities, CHRISTUS Mother Frances Hospital-Sulphur Springs is still able to accept patients. In fact hospitals in the region continue to be at less than the 15 percent identified by the...
CMFH-SS Has Updated Visitation Guidelines, Offers COVID-19 Vaccines
April 5, 2021 Business News By Holly Ragan, Senior Market Development, CHRISTUS Mother Frances Hospital – Sulphur Springs, [email protected] Sulphur Springs, Texas, April 5, 2021 – CHRISTUS Mother Frances Hospital – Sulphur Springs has ONE mission: To Extend the Health Ministry of Jesus Christ. Important Notice We are aware of the new order announced by Texas Governor Greg Abbott that would lift the statewide...
Texas To Expand COVID-19 Vaccine Eligibility To All Adults Starting March 29
DSHS To Launch Website Where People Can Register For COVID-19 Shot Through Some Public Health Providers Texas Department of State Health Services and Health and Human Services at 10 a.m. Tuesday, announced that the state’s vaccination plan is expanding again at the end of next week. All adults will be eligible to receive a COVID-19 vaccine in Texas beginning Monday, March 29. The Texas Department of State Health Services, in the...