USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...
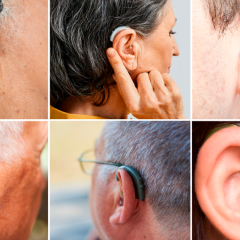
FDA Finalizes Rule Improving Access To OTC Hearing Aids
More Affordable Hearing Aids For Those With Mild To Moderate Hearing Impairment Could Be In Stores As Soon As Mid-October Today (Aug. 16, 2022), the U.S. Food and Drug Administration issued a final rule to improve access to hearing aids which may in turn lower costs for millions of Americans. This action establishes a new category of over-the-counter (OTC) hearing aids, enabling consumers with perceived mild to moderate hearing...
FDA Denies Authorization To Market JUUL Products
MDO: Currently Marketed JUUL Products Must Be Removed From US Market Today, the U.S. Food and Drug Administration issued marketing denial orders (MDOs) to JUUL Labs Inc. for all of their products currently marketed in the United States. As a result, the company must stop selling and distributing these products. In addition, those currently on the U.S. market must be removed, or risk enforcement action. The products include...
FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
Steps FDA Is Taking to Improve Specialty, Infant Formula Supply
FDA News Release Today (May 10, 2022), the U.S. Food and Drug Administration is providing an update on its work to increase the availability of infant and specialty formula products. On Feb. 17, the agency warned consumers not to use certain powdered infant formula products from Abbott Nutrition’s Sturgis, Michigan facility, and Abbott initiated a voluntary recall of certain products. Since that time, the agency has been working with...
FDA Proposes Product Standards Prohibiting Menthol Cigarettes, Flavored Cigars
Administration Hopes Change Will Help Prevent Youth Use, Significantly Reduce Tobacco-Related Disease and Death US. Food And Drug Administration April 28, 2022 Release Today (April 28, 2022), the U.S. Food and Drug Administration is announcing proposed product standards to prohibit menthol as a characterizing flavor in cigarettes and prohibit all characterizing flavors (other than tobacco) in cigars. These actions have the potential...