CMFH-SS COVID-19 Response Includes Entry Screenings, Virtual Medicine, Chatbot
CHRISTUS Mother Frances Hospital-Sulphur Springs Celebrates Doctor’s Day, Welcomes New Wound Care Associate, Prepares For Blood Drive A CMFH-SS News Release — CHRISTUS Mother Frances Hospital – Sulphur Springs has ONE mission: To Extend the Health Ministry of Jesus Christ. Happy National Doctor’s Day, a day celebrated to recognize the contributions of physicians to individual lives and communities. CMFH-SS appreciates you...
City Of Sulphur Springs Establishes Small Business Assistance Program In Response To COVID-19
Applications Available Online And Will Be Accepted Beginning At 8 a.m. July 8 Sulphur Springs City Council Tuesday night authorized the establishment of a small business assistance program in response to the COVID-19 pandemic. Qualifying small Sulphur Springs businesses can download the application starting Tuesday evening from the COVID-19 Business Resources menu on the City of Sulphur Springs’ website to seek up to $2,500 in...
USFDA Authorizes Bivalent COVID-19 Vaccines For Children Down To 6 Months of Age
FDA NEWS RELEASE — December 8, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age. “More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to...
Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines Authorized As Booster For Younger Kids
US FDA News Release, Oct. 12, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups. The Moderna COVID-19 Vaccine, Bivalent is authorized for administration at least two months following completion of primary or booster vaccination...
FDA Authorizes EAU Of Pfizer COVID-19 Single-Dose Booster For Kids 5-11 Years Old
US FDA news release – Tuesday, May 17, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 5 through 11 years of age at least five months after completion of a primary series with the Pfizer-BioNTech COVID-19 Vaccine. “While it has largely been the case that...
Alcohol Isn’t The Only Thing A Breath Test Can Detect; It Can Now Detect COVID-19 Too
Alcohol isn’t the only thing a breath test can detect; COVID-19 can now be detected in less than 3 minutes using a breath sample. Not having to stick a long cotton swab up the nose, have a blood sample drawn or cheek swab to check for the virus sounds great, just don’t expect to see the new diagnostic devices readily available to the average consumer anytime soon – or even some locations that previously conducted...
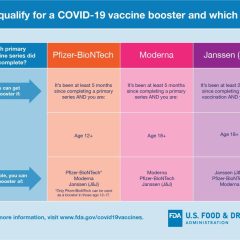
USFDA Authorizes Second Booster Dose Of Pfizer, Moderna COVID-19 Vaccines For Ages 50 And Up And Immunocompromised Individuals
Today (March 28, 2022), the U.S. Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This makes a second booster dose of both vaccines available...
Several Area Schools Closed Following COVID-19 Surge
Regardless of your opinions regarding COVID-19 and vaccinations, the virus has definitely taken a toll on local school districts this week, further taxing staff who were already feeling the stress caused as illnesses swept from classrooms to campuses and across districts. Many school districts in the area on Wednesday and Thursday announced plans for their schools will be closed through Martin Luther King Jr. Day, and are set to...
FDA Shortens Interval For Booster Dose of Moderna COVID-19 Vaccine to 5 Months
FDA News Release – Jan. 7, 2022 Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least 5 months for individuals 18 years of age and older. “The country is in the middle of a wave of the highly contagious omicron variant, which spreads more rapidly than...
FDA Acts To Expand Use Of Pfizer COVID-19 Vaccine
FDA Jan. 3, 2022 News Release Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to: Expand the use of a single booster dose to include use in individuals 12 through 15 years of age.Shorten the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months.Allow for a third primary...